O0925 Efficacy and safety of whole-body chlorhexidine gluconate (CHG) skin application, with or without emollient, in reducing bacterial skin colonisation of hospitalised low birth weight neonates: the NeoCHG trial
Background
Healthcare associated infections account for substantial neonatal in-hospital mortality. Chlorhexidine gluconate (CHG) whole body skin application may reduce sepsis by lowering bacterial colonisation density, although safety and the optimal application regimen is unclear. Emollients including sunflower oil may improve skin condition thereby reducing sepsis. This pilot trial aimed to inform which concentration and frequency of CHG, with or without emollient, would best balance safety and efficacy in a future pragmatic trial evaluating clinical outcomes.
Methods
Neonates in 2 hospital sites in South Africa and Bangladesh aged 1-6 days with gestational age ≥28 weeks and birth weight 1000-1999g were randomised in a factorial design to three different concentrations of CHG (0.5%, 1% and 2%), with or without emollient (sunflower oil) and on working days vs alternate working days. The control arm received neither product. Co-primary outcomes were efficacy and safety. Efficacy was measured as skin bacterial load in the nose, cervical skin folds and umbilicus (pooled swab), and peri-rectal area from randomisation to D3 ± 1 day and D8 ± 3 days. Safety was assessed via a modified neonatal skin condition score, assessing skin dryness, erythema and breakdown.
Results
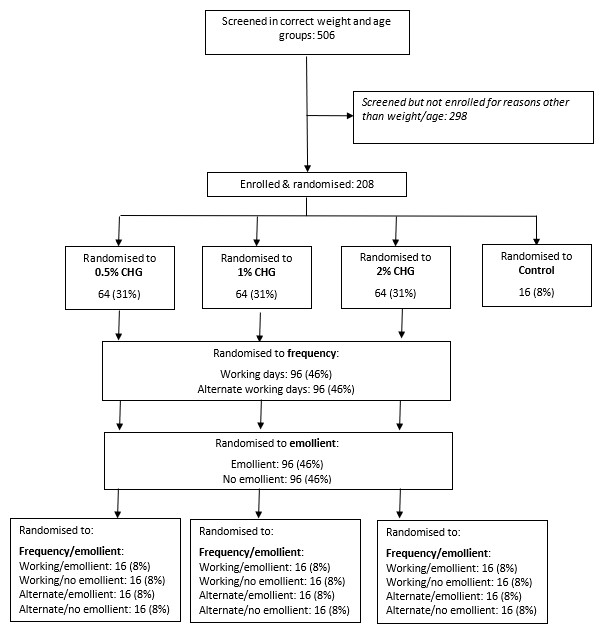
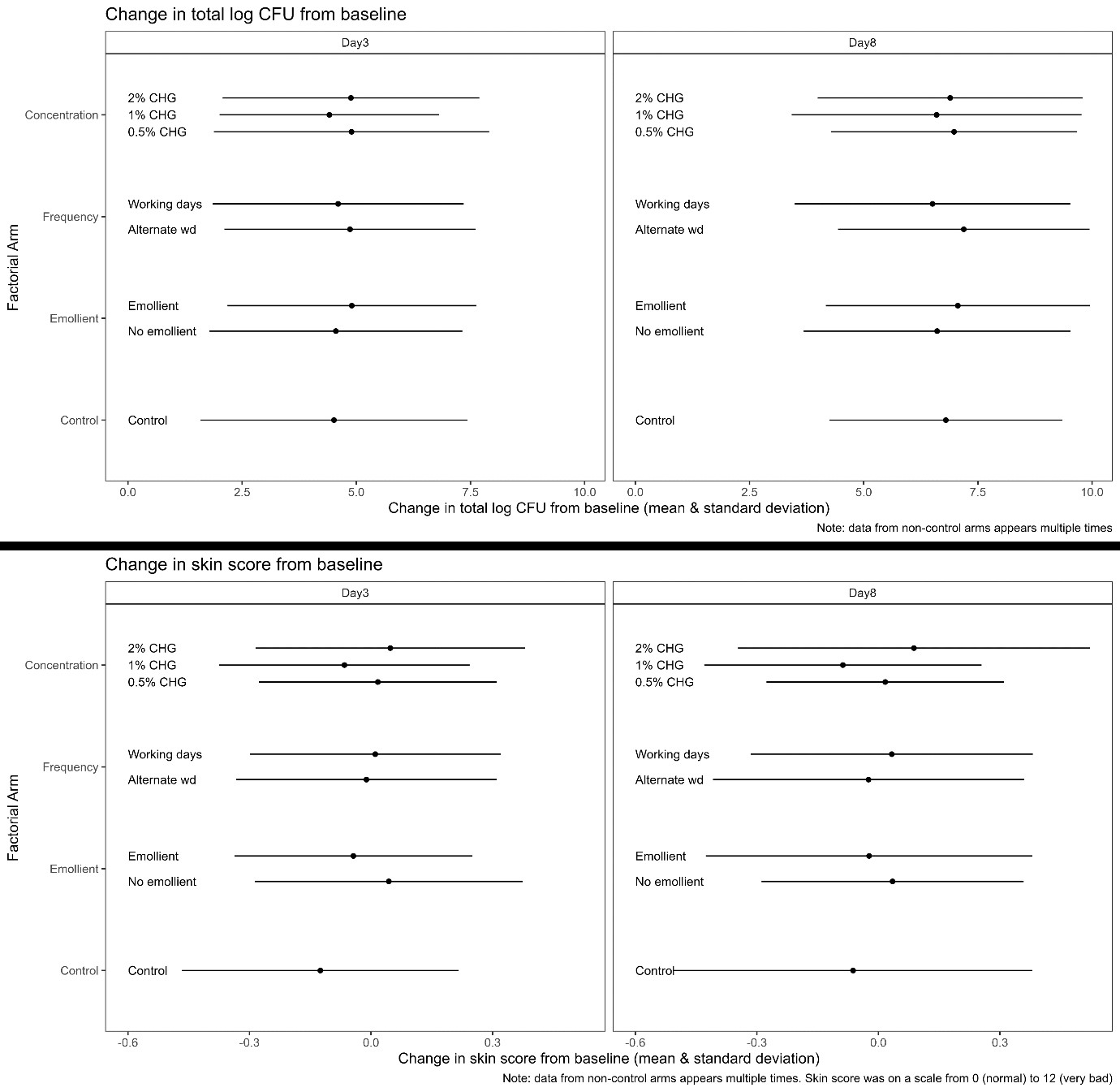
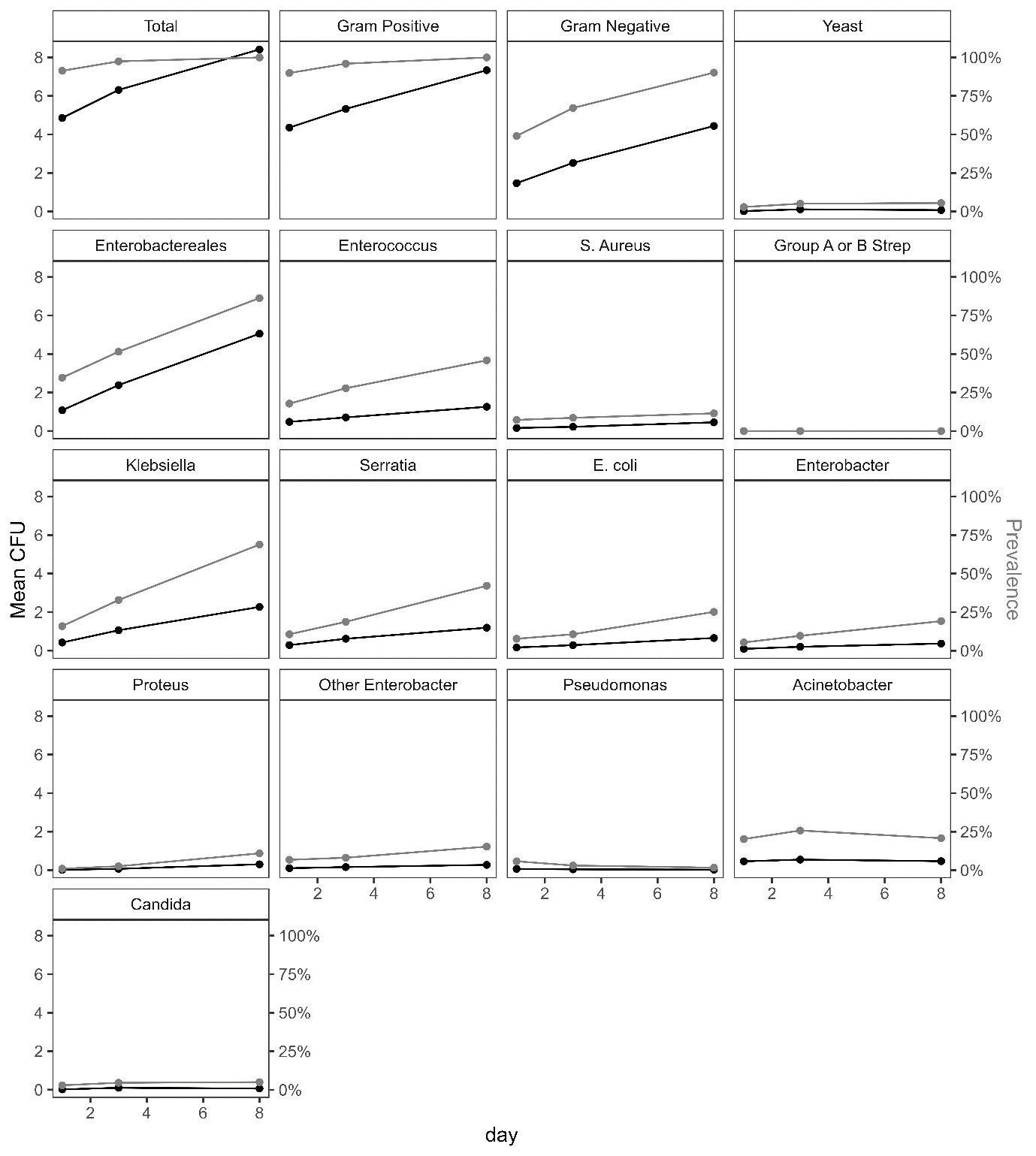
Between April 2021-January 2022, 208 infants were enrolled (figure 1). The average total log CFU was 4.86 (SD = 3.01) at baseline, 6.31 (SD = 3.10) at day 3 and 8.42 (SD = 2.62) at day 8. There was no convincing evidence of a difference between arms (figure 2), but a trend towards lower bacterial load with 1% CHG. Skin scores were low (lower=better), with no evidence of a difference between arms, although there was a trend towards better skin condition for infants <1.5kg receiving emollient. By day 8, 80% of neonates were colonised with Enterobacterales, and 1/3 and 1/10 with 3rd generation cephalosporin resistant and Carbapenem resistant Klebsiella spp respectively, regardless of trial arm (figure 3).
Conclusions
CHG +/- emollient could feasibly be evaluated in a larger RCT. There was no clear evidence to recommend a specific regimen, but no suggestion of benefit in increasing CHG concentration above 1%. There was rapid colonisation with Enterobacterales and frequent resistance, regardless of skin application regimen.
Conclusions
Case(s) description
Discussion
References